NF-κB is an important transcription factor for immune cell development and function and is regulated by IκB proteins. IκBNS is an unusual IκB protein and functionally poorly characterized. During the 2nd funding period, A 23 established that IκBNS-/- mice are resistant towards high-dose Listeria infection, hinting towards alterations in innate immunity. Indeed, we found extraordinarily high expression of IκBNS in macrophages/monocytes, neutrophils and NK cells using reporter mice. In the next funding period, we will decipher molecular and cellular functions of IκBNS in these cells using newly established conditional knockout mice that are currently being characterized. This includes IκBNS-dependent leucocyte migration during Listeria infection and functional characterization of target genes and microRNAs.
A 23 - The role of the atypical NF-қB inhibitory protein IқBNS in effector cells
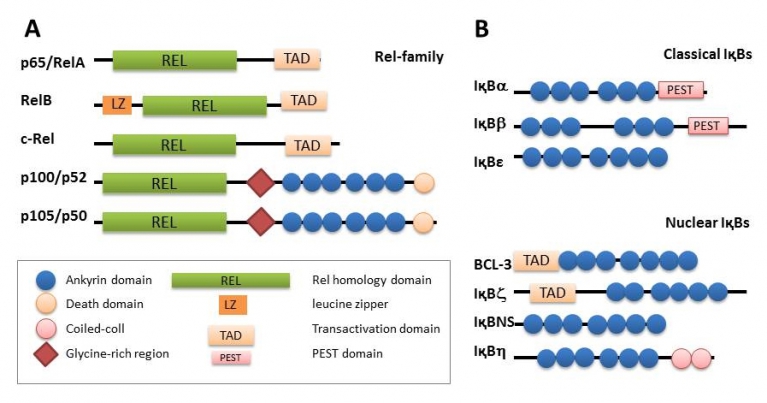
The Rel/NF-κB family
A) The Rel-family consists of five members. Their characteristic structural motif is the Rel-homology domain. RelA, RelB and c-Rel contain a transactivation domain, NF-κB transcription factors containing one of these subunits induce gene transcription. p52 and p50 originate from precursor proteins p100 and p105, respectively. Remarkably, these proteins contain ankyrin domains, the common structural motif of IκB proteins, which are proteolytically cleaved after activation of the NF-κB pathway.
B) The IκB family. The family of IκB proteins consists of the classical members IκBα, IκBβ and IκBε that are distinguished from the unusual members IκBζ, BCL-3 and IκBNS. All members share the ankyrin domain, which is responsible for interaction with the Rel-homology domain of NFκB subunits. In contrast to classical IκBs, unusual IκBs are restricted to the nucleus and not degraded after NFB activation. Moreover, they are inducible and are not only repressors but can also have activating properties, e.g. for IL-6 (IκBζ) and IL-2 (IκBNS). (Modified from Siebenlist et al. (2005) Nat. Rev. Immunol.)